
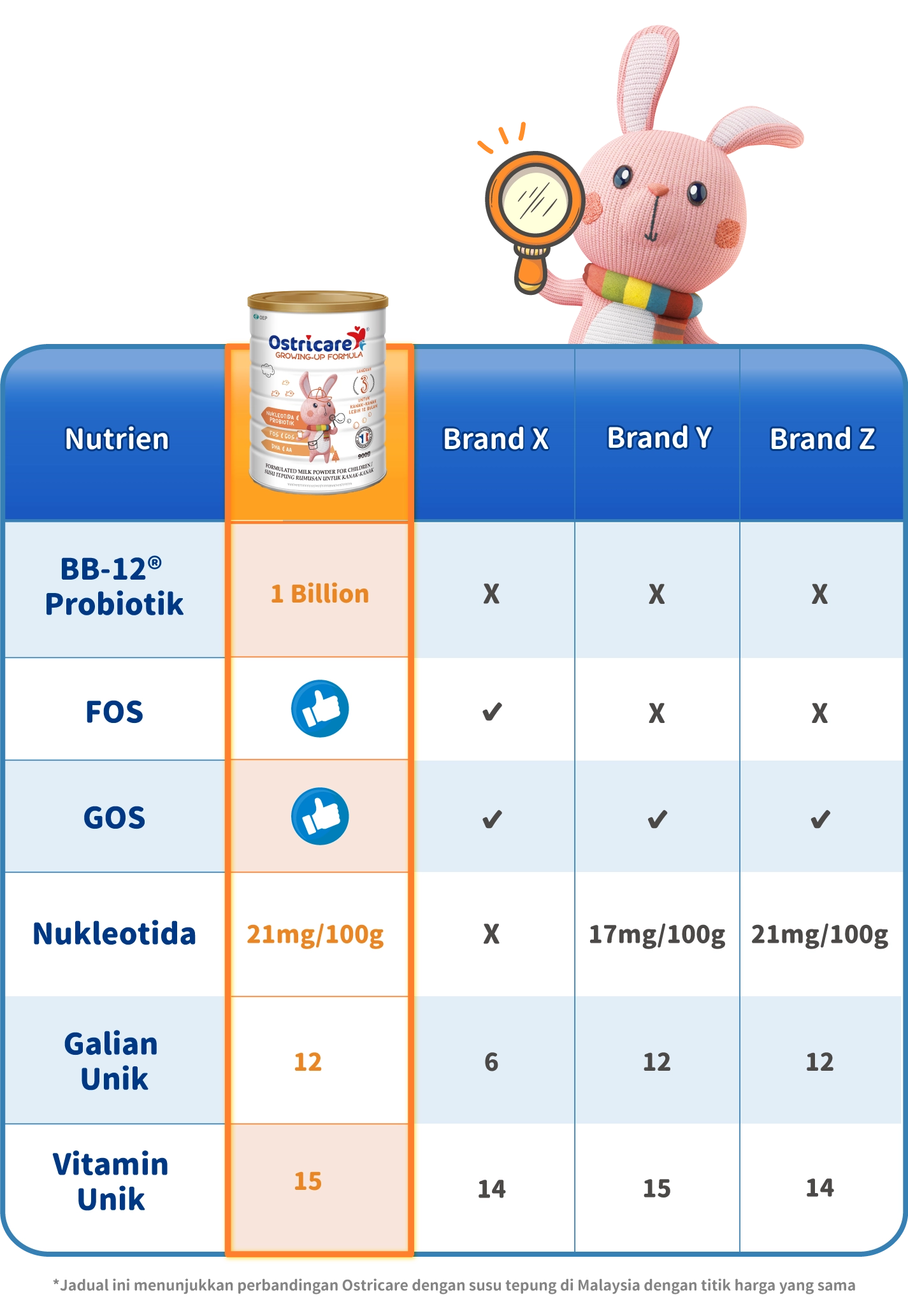
Susu formula Ostricare diperbuat daripada susu lembu tulen dari Perancis. Ia menawarkan dua ramuan unik, nukleotida dan probiotik BB-12® yang disokong oleh kajian klinikal untuk mengukuhkan pertahanan semula jadi kanak-kanak. Beri anak anda semua perlindungan, penjagaan dan tenaga untuk meneroka dunia tanpa sebarang kebimbangan!



BB-12® mengurangkan risiko jangkitan salur pernafasan1

BB-12® berkesan dalam mengurangkan tangisan dan ragam kanak-kanak yang didiagnosis
dengan kolik2

Probiotik BB-12® berkesan dalam meningkatkan imuniti semula jadi terhadap jangkitan saluran pernafasan dan menggalakkan kualiti tidur yang lebih baik untuk kanak-kanak.

Rantaian probiotik BB-12® dari Chr. Hansen menerima status Generally Recognized As Safe (GRAS) oleh Food and Drug Administration (FDA) di US untuk kanak-kanak dan populasi umum.8
Di Eropah, Bifidobacterium animalis telah dianugerahkan status Qualified Presumption of safety (QPS) sejak 2007 oleh European Food Safety Authority (EFSA)
- Status diberikan kepada tahap spesis.9











Resource:+
1.Jungersen et al. 2014. The Science behind the Probiotic Strain Bifidobacterium animalis subsp. lactis BB-12®. Microorganisms, 2(2), pp.92-110
2.Chen et al. 2021. Efficacy of Bifidobacterium animalis subsp. lactis, BB-12® on infant colic–a randomised, double-blinded, placebo-controlled study. Beneficial Microbes, 12(6), pp.531-540.
3.Vlieger et al. Tolerance and safety of Lactobacillus paracasei ssp. paracasei in combination with Bifidobacterium animalis ssp. lactis in a prebiotic-containing infant formula: a randomised controlled trial. British journal of nutrition, 102(6), pp.869-875.
4.Taipale et al., 2011. Bifidobacteriumanimalis subsp. lactis BB-12 in reducing the risk of infections in infancy. British Journal of Nutrition, 105(3), pp.409-416
5.Nocerino et al., 2020. The therapeutic efficacy of Bifidobacterium animalis subsp. lactis BB‐12® in infant colic: A randomised, double blind, placebo‐controlled trial. Alimentary pharmacology & therapeutics, 51(1), pp.110-120.
6.Isolauri et al., 2021. Nucleotides as optimal candidates for essential nutrients in living organisms: A review. Journal of Functional Foods, 82, p.104498.
7.Yau et al., 2003. Effect of nucleotides on diarrhea and immune responses in healthy term infants in Taiwan. Journal of pediatric gastroenterology and nutrition, 36(1), pp.37-43.
8.Food and Drug Administration. GRAS Notice Inventory > Agency Response Letter GRAS Notice No GRN 000049. 2002.
9.EFSA Panel on Biological Hazards (BIOHAZ). Statement on the update of the list of QPS-recommended biological agents intentionally added to food or feed as notified to EFSA 3: Suitability of taxonomic units notified to EFSA until September 2015. EFSA Journal. 2015;13:4331.




BB-12® mengurangkan risiko jangkitan salur pernafasan1

BB-12® berkesan dalam mengurangkan tangisan dan ragam kanak-kanak yang didiagnosis dengan kolik2



Rantaian probiotik BB-12® dari Chr. Hansen menerima status Generally Recognized As Safe (GRAS) oleh Food and Drug Administration (FDA) di US untuk kanak-kanak dan populasi umum.8
Di Eropah, Bifidobacterium animalis telah dianugerahkan status Qualified Presumption of safety (QPS) sejak 2007 oleh European Food Safety Authority (EFSA)
- Status diberikan kepada tahap spesis.9











Resource:+
1.Jungersen et al. 2014. The Science behind the Probiotic Strain Bifidobacterium animalis subsp. lactis BB-12®. Microorganisms, 2(2), pp.92-110
2.Chen et al. 2021. Efficacy of Bifidobacterium animalis subsp. lactis, BB-12® on infant colic–a randomised, double-blinded, placebo-controlled study. Beneficial Microbes, 12(6), pp.531-540.
3.Vlieger et al. Tolerance and safety of Lactobacillus paracasei ssp. paracasei in combination with Bifidobacterium animalis ssp. lactis in a prebiotic-containing infant formula: a randomised controlled trial. British journal of nutrition, 102(6), pp.869-875.
4.Taipale et al., 2011. Bifidobacteriumanimalis subsp. lactis BB-12 in reducing the risk of infections in infancy. British Journal of Nutrition, 105(3), pp.409-416
5.Nocerino et al., 2020. The therapeutic efficacy of Bifidobacterium animalis subsp. lactis BB‐12® in infant colic: A randomised, double blind, placebo‐controlled trial. Alimentary pharmacology & therapeutics, 51(1), pp.110-120.
6.Isolauri et al., 2021. Nucleotides as optimal candidates for essential nutrients in living organisms: A review. Journal of Functional Foods, 82, p.104498.
7.Yau et al., 2003. Effect of nucleotides on diarrhea and immune responses in healthy term infants in Taiwan. Journal of pediatric gastroenterology and nutrition, 36(1), pp.37-43.
8.Food and Drug Administration. GRAS Notice Inventory > Agency Response Letter GRAS Notice No GRN 000049. 2002.
9.EFSA Panel on Biological Hazards (BIOHAZ). Statement on the update of the list of QPS-recommended biological agents intentionally added to food or feed as notified to EFSA 3: Suitability of taxonomic units notified to EFSA until September 2015. EFSA Journal. 2015;13:4331.




